TMS Clinical Results
TMS Clinical Results
TMS Therapy was cleared by the FDA in 2008 after a decade of clinical trials. As such, the actual administering of TMS is very precise and well regulated. Since TMS Therapy was first introduced, the evidence for the clinical efficacy of treating patients in the prefrontal cortex for major depression spans more than 30 randomized, controlled trials in thousands of patients. Overall, these reports represent a comprehensive and consistent literature in which conclusions dramatically substantiate the effectiveness of TMS Therapy. Shown below are the TMS clinical results of three separate studies representing a total of 462 patients with treatment performed at over 40 different locations nationwide.
Study 1: Dunner 2014
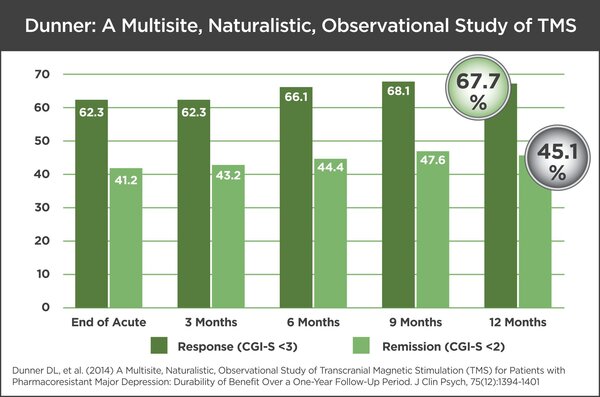
In a naturalistic clinical study in 257 patients who did not benefit from antidepressant medications, TMS Therapy offered durable effect over 1 year following acute treatment. The study was conducted at 42 different sites, of which 76% were in private clinical practices, 17% in academic medical centers, and 7% within nonacademic institutional settings. The distribution of practice types participating in the study mirrors those currently offering TMS Therapy in the United States. The TMS clinical results were impressive: 67.7% of patients responded to treatment and 45.1% reached remission.
While the results at the end of the initial treatment (end of acute phase) indicated a response rate of 62.3% and a remission rate of 41.2%, the results improved when compared 12 months later. The improvement is partially a result of “reintroduction treatments” that were reintroduced to 36.2% of patients (average of 16 treatments).
Study 2: Vaché 2013
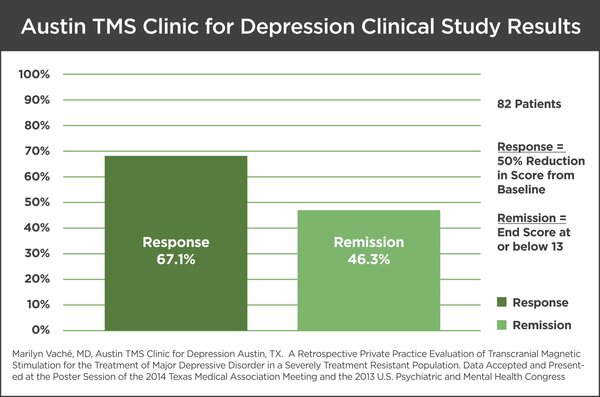
In this study, 82 patients were treated with TMS Therapy and evaluated using the IDS-SR30 symptom severity scale immediately before and after treatment. The mean IDS-SR30 symptom severity scale before treatment was 42.8 and reduced to 20.1 at the end of treatment. Fifty percent of the patients included in this evaluation had a prior history of hospitalization related to their depressive symptomatology. TMS sessions were conducted 5 days per week for a minimum of 2 weeks with a mean of 32.8 treatments per acute phase. The results were impressive with 55 of the 82 (67.1%) patients positively responding to TMS treatment and with 38 of 82 (46.3%) patients identified to be in remission. Response rates were determined based upon a minimum of a 50% reduction in symptom score compared to the baseline. No significant adverse events were experienced during or after treatment.
Study 3: Cress 2015
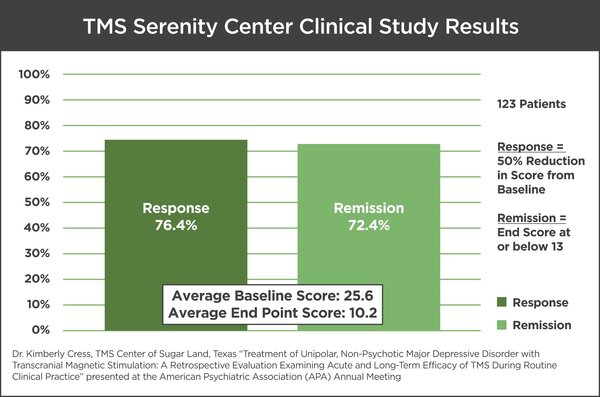
In this study, 123 patients with unipolar, non-psychotic MDD who had failed to receive benefit from prior antidepressant treatment were given TMS Therapy using the NeuroStar TMS Therapy System (Neuronetics, Inc., Malvern, PA, USA). Patients were treated daily (five days a week for approximately four to six weeks). The study’s primary outcome of interest was the change in depressive symptoms utilizing the Beck Depressive Inventory Scale (BDI II). The mean baseline score was 25.6, indicating moderate depression with a mean end point score of 10.2 by the end of the study, indicating remission.
Overall, 89 out of the 123 patients (72.4 percent) achieved remission of symptoms during acute treatment. There were no serious adverse events reported. From this patient population, 10 patients who achieved remission in the acute phase of TMS were evaluated over the course of four years, with over 80.0 percent of patients maintaining remission from their depression.
*TMS Therapy has also undergone, or is currently undergoing, hundreds of clinical trials for the treatment of many other disorders, including: ADD, PTSD, bi-polar disorder, autism, chronic pain, epilepsy, obesity, smoking cessation, Fibromyalgia, Parkinson’s and Alzheimer’s. Many leading medical universities and institutions are involved in these clinical trials, including Columbia, Duke, Harvard, Johns Hopkins, Mayo Clinic, Stanford, UCLA, University of Pennsylvania, and Walter Reed National Military Medical Center.
For more information on past and current clinical trials involving Transcranial Magnetic Stimulation, visit the U.S. National Institutes of Health website.
